IISER MOCK TEST 3
The IISER Aptitude Test is a crucial part of the admission process. It is designed to test the candidate’s knowledge and understanding of basic science subjects. The exam is usually held in June, and it consists of multiple-choice questions from Physics, Chemistry, Mathematics, and Biology.
The syllabus for the IISER Aptitude Test covers topics from the higher secondary curriculum. The exam pattern includes four sections, each focusing on one of the core subjects. It’s essential to understand the weightage and structure of the exam to strategize your preparation effectively.

Q.1 A refrigerator absorbs 2000cal of heat from ice trays. If the coefficient of performance is 4 , then work done by the motor is
A. 2100/
B. 4200/
C. 8400/
D. 500/
Q2. A fixed mass of a gas is first heated isobarically to double the volume and then cooled isochorically to decrease the temperature back to the initial value. By what factor would the work done by the gas decreased, had the process been isothermal?
A. 2
B. 1/2
C. ln 2
D. ln 3
Q3. If p represents radiation pressure, C represents the speed of light, and Q represents radiation energy striking a unit area per second, the non zero integers x, y, and z such that Px Sy C2 is dimensionless, find the values of X, Y, and 2 ?
A. x = 1, y = 1, z = -1
B. x = 1, y = -1, z = 1
C. x = -1, y = 1, z = 1
D. x = 1, y = 1, z = 1
4. The ratio of 1kWℎ (kilowatt-hour) to 1MeV (million electron volt) is
A. 2.25 × 1017
B. 2.25 × 1019
C. 2.25 × 1023
D. 2.25 × 4.4 × 10 9
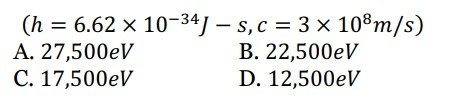
A. Energy and linear momentum
B. Linear and angular momentum
C. Energy and angular momentum
D. None of the above
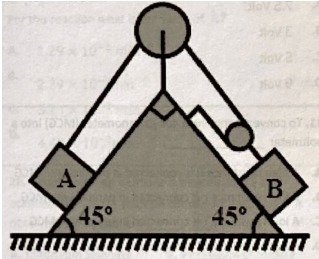
7. Two blocks A and B of mass 10kg and 40kg are connected by an ideal string as shown in the figure. Neglect the masses of the pulleys and the effect of friction in the pulleys and between the blocks and the inclines. Then

Q 8. A small block of mass M moves with velocity 5ms -1 towards n another block of the same mass M the velocity of the particle when its acceleration is zero?
A. −9ms -1
B. −12ms -1
C. −3ms -1
D. 42ms -1
Q 9. The work that must be done in lifting a body of weight p from the surface of the
earth to a height ℎ is .

Q 10. A car has a fresh storage battery of EMF 12V and internal resistance 5 × 10-2 If the starter motor draws an electric current of 90A what is the terminal voltage of the battery when the starter is on?
A. 7.5 Volt
B. 3 Volt
C. 5 Volt
D. 9 volt
Q.11 To convert a moving a coil galvanometer (MCG) into a voltmeter
A. A high resistance R is connected in
parallel with MCG
B. A low resistance r is connected in
parallel with MCG
C. A low resistance r is connected in
series with MCG
D. A high resistance R is connected in
series with MCG
Q.12 Which of the following has the maximum resistance?
A. Voltmeter
B. Millivoltmeter
C. Ammeter
D. Milliammeter


Q 14. For a particle moving in a straight line, the displacement of the particle at time T is given by S = t3 3 − 6t 2 + 3t + 4. What is the velocity of the particle when its acceleration is zero?
A. −9ms -1
B. −12ms -1
C. −3ms -1
D. 42ms -1
Q 15. A body is projected with initial velocity of (6i + 8i) ms -1 . The horizontal range is
A. 9.6m
B. 14m
C. 50m
D. None of these
Chemistry
Q 16. The density of O2 is 16kg/m3 at nRT. At what temperature its density will be 14kg/m3 ? consider that the pressure remains constant.
Q 17. Identify the correct statement regarding a spontaneous process.
A. Lowering of energy in the reaction process is the only criterion for spontaneity
B. For a spontaneous process in an isolated system, the change in entropy is positive
C. Endothermic processes are never spontaneous
D. Exothermic processes are always spontaneous
Q 18. The free energy change for the following reactions are given below
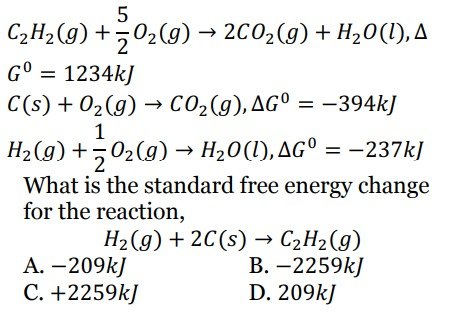
Q 19. Electrons are emitted with zero velocity from a metal surface when it is exposed
to radiation of wavelength 6800Å work function (WO) of the metal.
A. 2.922 × 10 -19
B. 2.922 × 10 -18
C. 2.922 × 10 -17
D. 2.922 × 10 -16
Q 20. Which of the following hydrogen bonds are strongest in the vapor phase?
A. HF…..HF
B. HF… HCL
C. HCL … HCL
D. HF … HI
A. 10%
B. 5.38%
C. 20%
D. 8.3%
Q 22. Study the following table.
Compound (Molecular weight)
II. NO2 (46)
IV. SO2 (64)
Weight of Compound (in g) taken
4.4
2.3
6.8
1.6
Which two compounds have the least weight of oxygen? (molecular weights of compounds are given in brackets).
A. II and IV
B. I and III
C. I and II
D. III and IV
Q 23. Which anion is the weakest base?

Q 24. Which of the following compound will undergo electrophilic substitution more readily than benzene?
A. Nitrobenzene
B. Benzoic acid
C. Benzaldehyde
D. Phenol
For this reaction what is the value of A ?
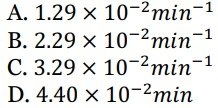
Q26. When hydrogen peroxide is treated with
a cold acidifled K2 Cr2 O7 solution
containing a blue color is obtained. This
is due to
A. Chromium Sulphate
B. Potassium Chromate
C. Perchromic Acid
D. Chromium Trioxide
A. +7
B. -1
C. +5
D. +1
Q 28. The number of P − O − P and P − O − H bonds present respectively in the pyrophosphoric acid molecule are
A. 1,2
B. 2,2
C. 1,4
D. 1,8
Q 29. Direction: For the Assertion (A) and Reason (R) below, choose the correct alternative.
Assertion: Nitrogen and Oxygen are the main components in the atmosphere but these do not react to form oxides of nitrogen. Reason: The reaction between nitrogen and oxygen requires a high temperature.
A. Both the Assertion and Reason are incorrect.
B. Both Assertion and Reason are correct and the Reason is the correct explanation for the Assertion.
C. Both Assertion and Reason are correct, but the Reason is not the correct explanation for the Assertion.
D. The Assertion is incorrect but the Reason is correct.
Q 30. Which of the following will convert cyclohexene into cis-1,2- cyclohexanediol ?
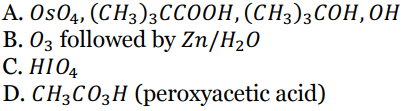
Mathematics

Biology
46. The complex formed by a pair of synapsed homologous chromosomes is called
A. Equatorial plate
B. Kinetochore
C. Axoneme
D. Bivalent
A. Amyloplast
B. Chloroplast
C. Chromoplast
D. Aleuroplast
Q 48. Which of the following may occur in absence of ephagy from residual bodies?
A. Exocytosis
B. Autodigestion
C. Polynephritis
D. Autolysis
Q 49. Nicotlana sylvestris flowers only during long days and N. tobacum flowers only during short days. If raised in the laboratory under different photoperiods, they can be induced to flower at the same time and can be cross- fertilized to produce self-fertile offspring. What is the best reason for
considering N. sylvestris and N .tobacum to be separate species?
A. They are physiologically distinct
B. They are morphologically distinct
C. They cannot interbreed in nature
D. They are reproductively distinct
Q50. Taxonomic hierarchy refers to
A. step-wise arrangement of all categories for classification of plants and animals
B. a group of senior taxonomists who decide the nomenclature of plants and animals
C. a list of botanists or zoologists who have worked on the taxonomy of a species or group
D. classification of a species based on the fossil record
Q51. Which of the following type of pollination is present in bombax?
A. Malacophilous
B. Ornithophilous
C. Anemophilous
D. Entomophilous
Q52. The bacterium, Bacillus thuringiensis, is widely used in contemporary biology as
A. classification of a species based on the fossil record
B. source of industrial enzyme
C. insecticide
D. indicator of water pollution
Q53. The reason for soil pollution is
A. Ozone
B. Aerosol
C. PAN
D. Acid rain
Q54. Pneumatophore is present in
A. Bryophyllum
B. Rhizophora
C. Sonneratia
D. Mangrove
Q55. The Fusion of two dissimilar gametes is called as
A. Fertilization
B. Pollination
C. Self Pollination
D. Self Fertilization
Q56. The type of Corolla present in Datura is
A. Campanulate
B. Wheel-shaped
C. Tubular shaped
D. Infundibuliform
Q57. Which of the following statement is incorrect w.r.t Cyclic photophosphorylation?
A. It is performed by Photosystem I independently
B. It not only connected with ATP synthesis, but also with synthesis of NADPH+ H+
C. The system does not take part in photosynthesis except in certain bacteria
D. In occurs mostly in stoma lamellae membrane
Q58. During closed stomata, which of the following enzyme can perform photosynthesis?
A. Phosphoenolpyruvate carboxylase
B. Phosphoglycerate
C. Triose phosphate
D. Ribulose 1-5 bisphosphate
Q59. Which of the following statement is incorrect w.r.t polymorphism?
A. Polymorphism in DNA sequence is the basis of genetic mapping in human genome as well as DNA fingerprinting
B. Polymorphism at genetic level arise due to mutation
C. Satellite DNA probe showing high degree of polymorphism is basis of DNA fingerprinting
D. Allelic sequence variation is described as DNA polymorphism if less than one variant at locus
occurs in human population with frequency less than 0.01
Q60. When both the alleles are equally expressed in hybrid, the phenomenon is known as
A. Partial dominance
B. Complete dominance
C. Codominance
D. Incomplete dominanc
Answer Key
Q. | Ans. |
1 | A |
2 | C |
3 | B |
4 | B |
5 | A |
6 | C |
7 | A |
8 | A |
9 | C |
10 | A |
11 | D |
12 | A |
Q. | Ans. |
13 | D |
14 | A |
15 | A |
16 | B |
17 | B |
18 | B |
19 | A |
20 | A |
21 | B |
22 | A |
23 | B |
24 | D |
Q. | Ans. |
25 | A |
26 | C |
27 | A |
28 | C |
29 | B |
30 | A |
31 | B |
32 | A |
33 | A |
34 | C |
35 | B |
36 | D |
Ans. | |
37 | B |
38 | C |
39 | C |
40 | D |
41 | A |
42 | A |
43 | D |
44 | A |
45 | D |
46 | D |
47 | B |
48 | C |
Q. | Ans. |
49 | C |
50 | A |
51 | C |
52 | C |
53 | D |
54 | B |
55 | A |
56 | D |
57 | B |
58 | A |
59 | D |
60 | C |
- KCET 2019 Physics Paper with Solutions
- KCET 2019 Chemistry Paper with Solutions
- KCET 2019 Maths Paper with Solutions
- KCET 2019 Biology Paper with Solutions
- KCET 2020 Chemistry Paper with Solutions
- KCET 2020 Physics Paper
- KCET 2020 Biology Paper with Solutions
- KCET 2020 Mathematics Questions Paper
- KCET Biology Examination 2021 Paper
- KCET – 2021 TEST PAPER
- IISER MOCK TEST – 4
- IISER MOCK TEST – 3
- IISER MOCK TEST – 2
- IISER MOCK TEST – 1
- KCET Mathematics- 2021 Test Paper
- KCET Chemistry – 2021 TEST PAPER
- KCET Physical – 2021 TEST PAPER
- KCET Biology Examination 2021 Paper
- KCET 2020 Mathematics Questions Paper
- KCET 2020 Biology Paper with Solutions
- KCET 2020 Physics Paper
- KCET 2020 Chemistry Paper with Solutions
- KCET 2019 Biology Paper with Solutions
- KCET 2019 Chemistry Paper with Solutions
- KCET 2019 Maths Paper with Solutions
